3d or 4s higher energy
Solve any question of Structure of Atom with-. In fact the orbital energies depend on the existence of other electrons.

Clarifying Electron Configurations Chemical Education Xchange
For most transition metals its the latter that wins out.

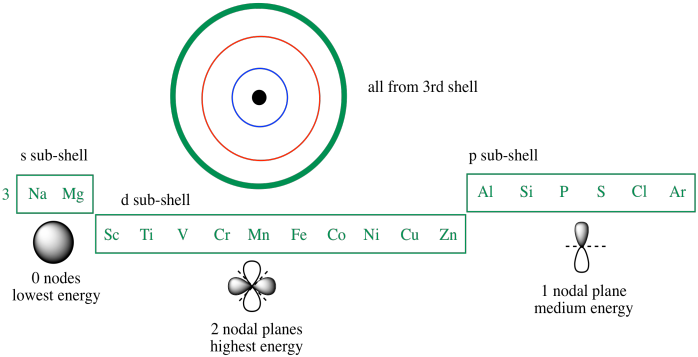
. Lower orbital energy but higher repulsion vs. Just as the area of a sphere increases when you increase its radius so too do the number of electrons increase as you increase the energy level. If two subshells or orbitals have the same nl value the subshell or orbital with lower n value will have lower energy.
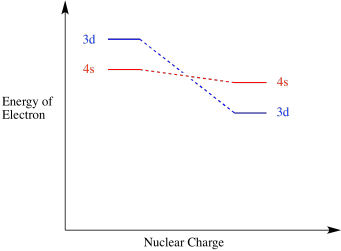
The oddity is the position of the 3d orbitals which are shown at a slightly higher level than the 4s. We know that the 4s electrons are lost first during ionization which means that they are of a higher energy level than the 3d orbital since the highest electrons are found farthest away from the nucleus thus are lost first during ionization. Browse discover thousands of brands.
We say that the 4s orbitals have a lower energy than the 3d and so the 4s orbitals are filled first. Answer 1 of 4. Answer 1 of 2.
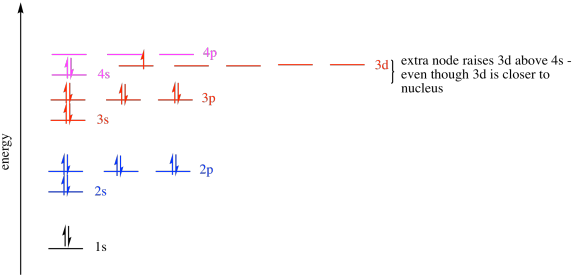
1s 2s 2p 3s 3p 4s 3d 4pWhy do electron configurations have 3d orbitals out of order from the rest. As it turns out for the first row of transition metals the 4s sublevel has higher energy than the 3d sublevel which is contrary to what we would expect from an Aufbau diagram. Thus in accordance to Aufbaus rule given statement is false.
For 4s4p3d the nl value is 4 04415325 respectively. This is discussed in detail here as well. The electrons lost first will come from the highest energy level furthest from the influence of the nucleus.
So the electron configuration for chromium is written in order of increasing energy with the 4s sublevel having the highest energy and written at the end. Read customer reviews find best sellers. In an atom with only one electron Hydrogen the 4s is not at lower energy than the 3d.
2 on a question Is 4s or 3d higher in energy. Ad Enjoy low prices on earths biggest selection of books electronics home apparel more. Energy is directly proportional to nl value.
If you are interested a detailed explanation for chromiums 3d5 4s1 configuration is here and an argument that does NOT. In multielectron atoms it is. Thus 4s has the least energy.
3d or 4s which has higher energywhy. This means that the 4s orbital which will. Share with your friends.
View Full Answer chocoboy has given the right answer. Electrons fill lower energy shells first before filling higher energy shells. So in order of decreasing energy starting at energy le.
My confusion arises when I look at the explanation on a website attached below it says that because ionization energy always removes the electron from the highest which mean that first removed electron is the last electron being put in the orbital so I conclude that 4s is higher than 3d in transition element. We know that the 4s electrons are lost first during ionisation. A new added electron would be affected by the hateful electrons trying to push it away.
However as soon as you form a transition metal ion the 3d orbitals plunge in energy even further and any electron that would have previously been in the 4s orbital. Through this observation we put the 3d orbital before the 4s orbital when writing electron orbitals. Here we break down how radial and angular nodes create a s.
So the 4s orbital must have a higher energy than the 3d orbitals. Its easy to imagine why. Higher orbital energy but lower repulsion.
This is because the electrons repel each other to a degree determined by the shape of the orbital they are in. I found a great online chat room there I met many good friends who are very friendly and. The 4s orbitals are usually higher in energy than the 3d specifically Sc through Kr but some textbooks dont acknowledge or recognize that Ca is the border past which the orbital energies switch.
3d has higher energy because as per nl rule 3d -- n 3 l2 nl 5 4s -- n4 l0 nl 4 54 3d4s. So we cannot say absolutely that the 4s orbital has lower energy level than 3d because it depends on the occupied orbitals. In the end its a trade-off.
3d orbital has greater energy than 4s because its nl value is 325 which is more than nl value for 4s404 orbital.

The Last Electron Of Potassium Enters Into 4s Orbital Rather Than 3d Tuition Tube
Why Is 4s Orbital Filled Before 3d Quora

Among The 3d And 4s Subshells Which Has Higher Energy

4s Vs 3d Which Orbital Is Higher In Energy Mr Khemistry

If 4s Orbitals Are Higher In Energy Than 3d Orbitals Then Why Do Electrons Fill Up In 4s Before Filling Up In 3d Quora
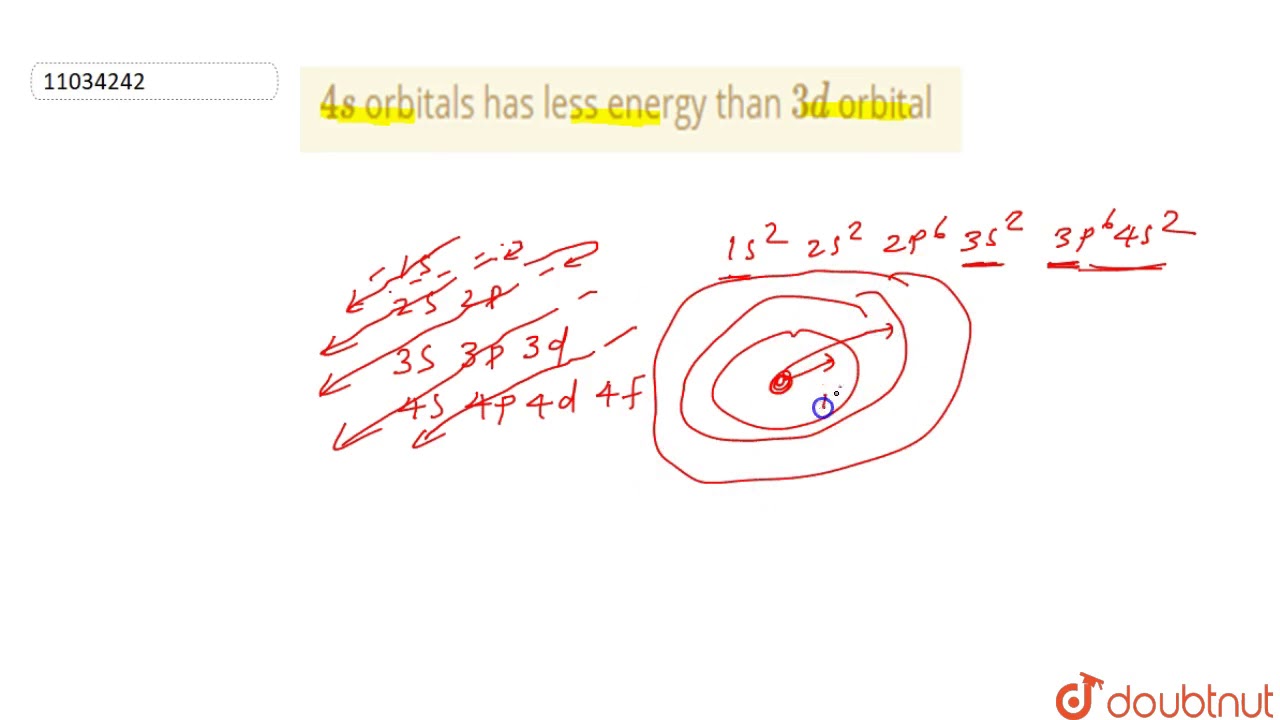
4s Orbitals Has Less Energy Than 3d Orbital Youtube
Why Is 4s Orbital Filled Before 3d Quora

If 4s Has A Lower Energy Level Why Are 3d Orbitals Written First Quora
Why Are Electrons In The 3d Orbital In A Higher Energy State Although Electrons In The 4s Orbital Are Further Out Socratic

The Decreasing Order Of Energy Of The 3d 4s 3p 3s Orbital Is Youtube

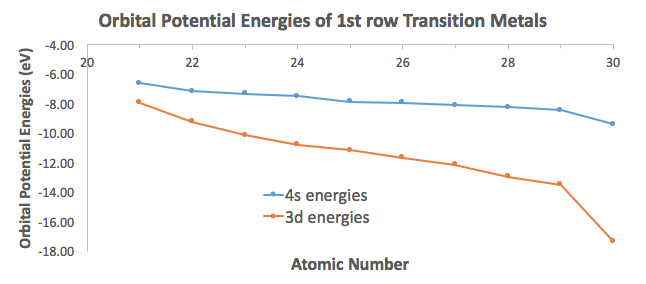
Variation Of 4s And 3d Orbital Energies As A Function Of Z Atomic Download Scientific Diagram
Why Is 4s Orbital Filled Before 3d Quora
Why Does The 3d Orbital Have A Lower Energy Than The 4s Orbital After The Element Scandium And Not Before Quora

Physical Chemistry Why Do 3d Orbitals Have Lesser Energy Than 4s Orbitals In Transition Metals Chemistry Stack Exchange
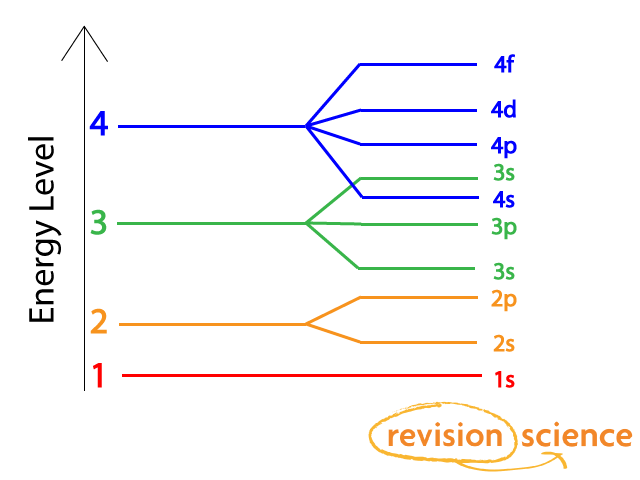
Electrons Filling Subshells And Orbitals A Level Chemistry Revision

Physical Chemistry Why Do 3d Orbitals Have Lesser Energy Than 4s Orbitals In Transition Metals Chemistry Stack Exchange